Cell and Gene Therapy (CGT) are highly specialized areas experiencing tremendous growth and opportunity in the biopharmaceutical industry. The war for talent in the CGT space, however is fierce and only going to escalate as more companies jump into this arena.
To put this into perspective, The Center for Biologics Evaluation and Research (CBER/FDA) has over 800 active gene therapy INDs in its pipeline for review. [1] MIT has predicted the number of new launches of cell and gene therapies is expected to reach between 40 and 60 launches (Figure 1), with 15-30 launching within the next five years. [2] The Alliance for Regenerative Medicine Report 2018 states that there are >900 companies currently exploring cell and gene therapies. [3] McKinsey estimates that Cell and Gene Therapy market will have total market of up to ~$40B by 2024. [4]
This explosive growth is putting tremendous pressure on CGT companies to find the right talent to advance their pipelines of these new therapies towards approval and commercial supply.
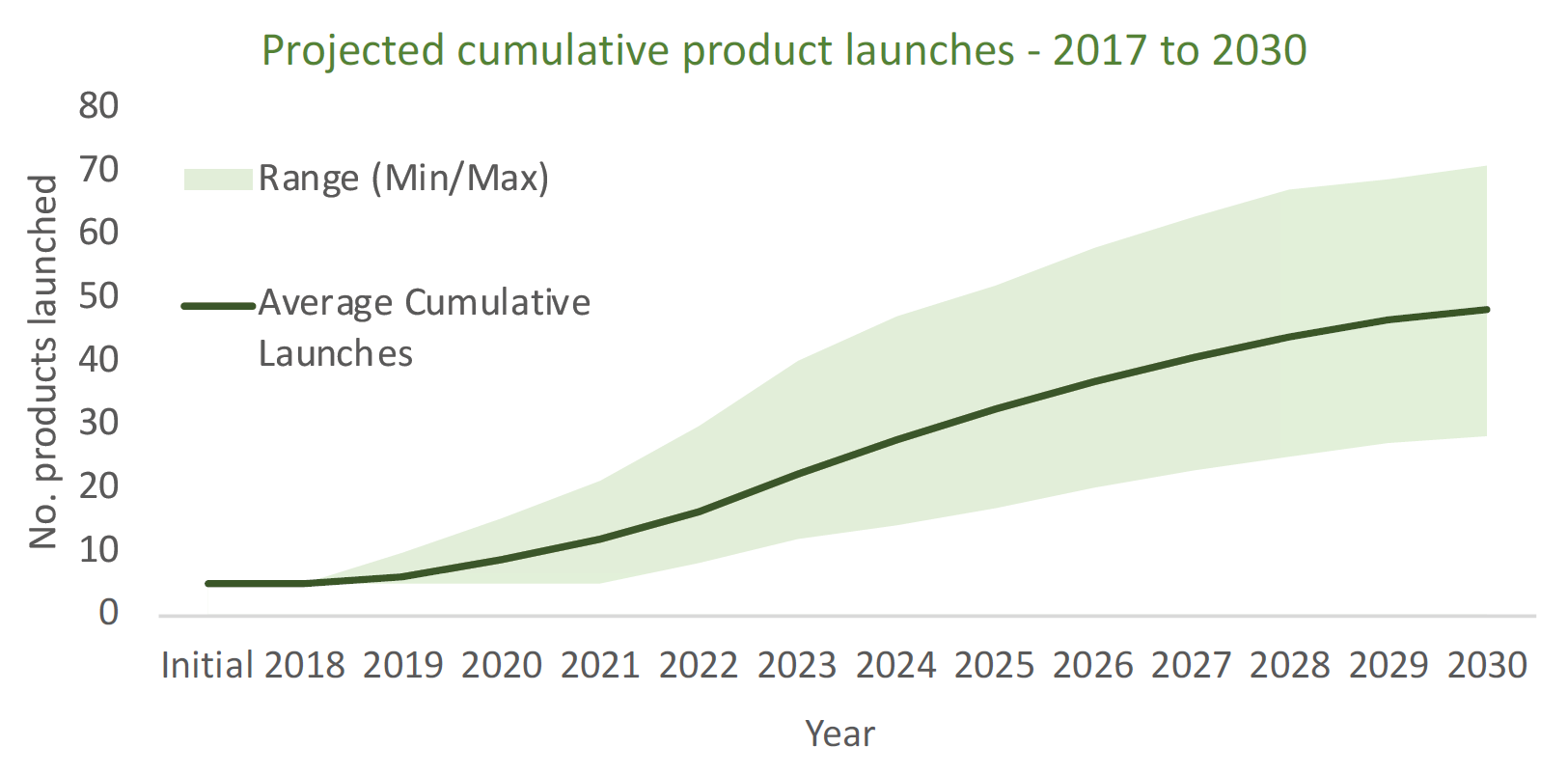
Figure 1: MIT NEWDIGS Research Brief 2018 Launches
Where is the Talent War?
The war for talent is mostly affecting the CGT space in three (3) major areas: process development, manufacturing and senior management. We review these areas below.
Process Development
Currently, the processes for making viruses in the pre-clinical stage are limited in terms of scalability. [5] New and improved processes are needed to provide efficient translation of early stage therapies into commercial-scale manufacturing.
For CGT companies to meet the rising demands, it is imperative to have experienced process development scientists in place to translate research-based processes into scalable processes for clinical and commercial manufacturing. [6] However, the roles are many and the experienced scientists are few.
Due to the talent shortage, the search for qualified candidates can be long and arduous. CGT companies are finding it extremely difficult to hire qualified and experienced professionals in process development across various levels. These include upstream and downstream scientists, cell line development specialists and senior engineers.
Manufacturing
At present, producing the essential components required for CGT, like viral vectors for gene therapy, is an extremely complex and expensive process. Currently these viruses need to be specially made in specialized facilities, drastically different than the traditional small molecule production facilities. [7]
GE Healthcare Biopharma [Fig.2] outlines key factors and capabilities needed to de-risk and successfully manufacture and provide to a greater number of patients over time. [8]. These evolve from the current highly-manual processes, to orchestrated supply chains, to global manufacturing and distribution systems. CGT companies are racing to find and on-board experts from a very limited talent pool with the experience to establish these yet-to-be developed functions and capabilities.
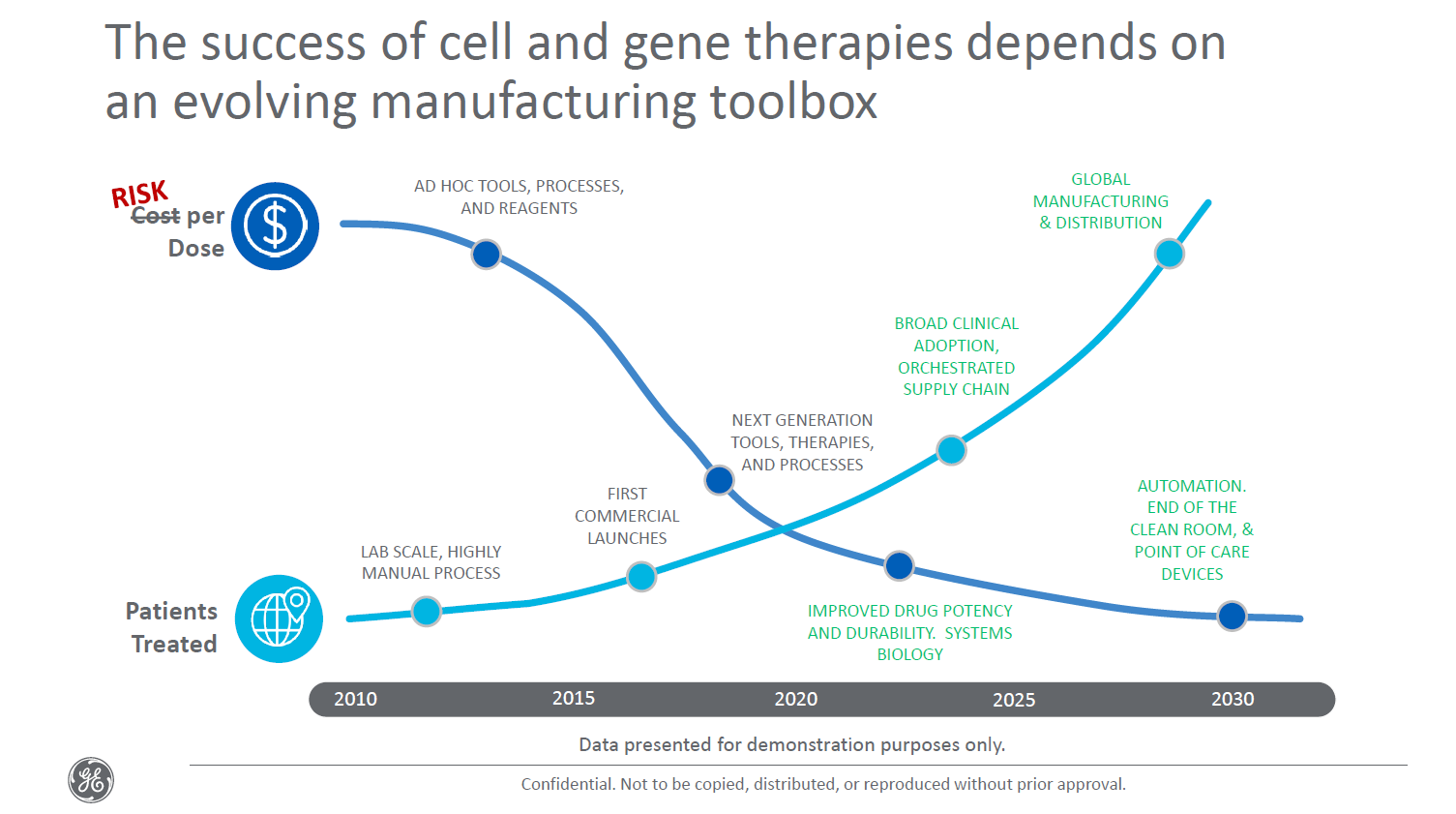
Figure 2: GE Healthcare Biopharma
Winning platforms, however, have yet to be chosen as testing and optimization continues. For cell therapies to be commercialized, efficient manufacturing infrastructure will need to be put in place. New technologies and next-generation techniques will help to create automated, closed systems that ensure both sterility and scalability in manufacturing of these cell therapies. Until these can be developed, tested and reliable, multiple manual methods will need to be used to produce different viral vectors and cell therapies. [9]
Both this lack of chosen platforms and the small number of experienced experts in manufacturing are serious challenges for the industry. As a result, many companies do not have the facilities, nor extensive expertise required to produce these viruses on a commercial scale. [6]
Senior-Level Management
Again, because the industry is young, there is a lack of seasoned managers with real, hands-on experience from end-to-end to step into fill these important strategic and tactical positions.
In order for CGT companies to attract the right candidate(s), they must be willing to pull out all the stops when offering compensation packages to secure these in-demand professionals. Some annual salaries have been shown to be approaching $300k and above
The Benefit of Using a Specialist Recruitment Agency
When working with Regional Personnel, clients benefit from our inside knowledge of pharmaceuticals, because we come from pharmaceuticals. We bring our deep technical expertise and pharmaceutical networks, developed over 30+ years, to every partner search.
Start a conversation with us: [email protected].
[1] Peter Marks MD, PhD; FDA CBER Presentation, BioNJ’s Manufacturing Briefing (Sept. 2019) [2] MIT NEWDIGS Research Brief 2018F210-v027-Launches [3] Alliance for Regenerative Medicine, State of the Industry, 2018, https://alliancerm.org/press-release/the-alliance-for-regenerative-medicine-releases-q1-2018-data-report-highlighting-sector-trends-and-metrics/ [4] McKinsey & Co. Presentation, BioNJ’s Manufacturing Briefing (Sept. 2019) [5] [6] Sargent, Brandy et al. “Key Considerations for Gene Therapy Commercialization.” Cell Culture Disk. 12 Sept. 2018, https://cellculturedish.com/key-considerations-for-gene-therapy-commercialization/ [7] “Demand Exceeds Supply in Cell & Gene Therapy Workforce.” Absorption Systems. 16 Sept. 2019, https://www.absorption.com/kc/demand-exceeds-supply-in-cell-gene-therapy-workforce/ [8] GE Healthcare Biopharma Presentation, BioNJ’s Manufacturing Briefing (Sept. 2019) [9] Hsu, Felix. “Driving Growth of the Cell and Gene Therapy Sector.” Pharma’s Almanac. 12 Mar. 2019, https://www.pharmasalmanac.com/articles/driving-growth-of-the-cell-and-gene-therapy-sector
James F. Lynch PhD, MBA – Regional Personnel Services ©2019